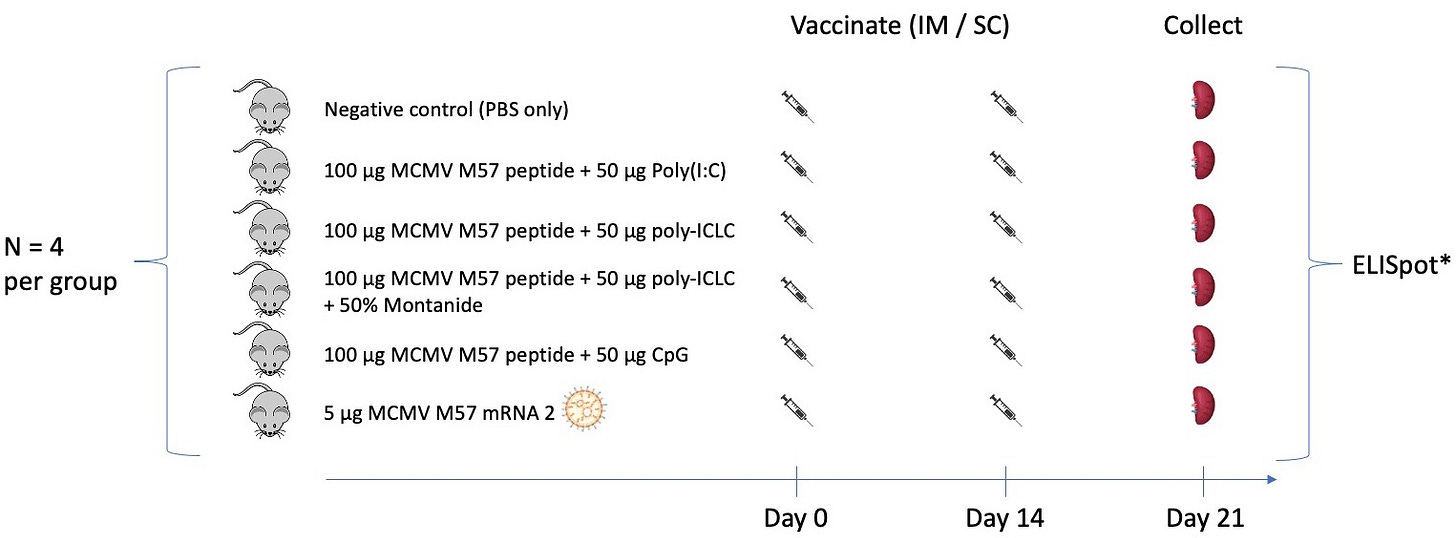
Potency of peptide + polyIC-LC +/- montanide vs CpG and mRNA/LNP for vaccination in C57Bl/6 mice.
Experiments here were planned, done, and reported by Jess Alley and Maria Sambade, with assistance from Blake Inabinett
In a prior experiment, we tested multiple vaccine formulations for capacity to elicit T cell responses in C57Bl/6 mice using a MHC class I-restricted murine cytomegalovirus epitope (M57) and an ELISPOT readout for interferon gamma production. We found that CpG (a TLR9 agonist) was the most potent peptide vaccine adjuvant tested, and that mRNA LNP vaccination was as potent as peptide + CpG vaccination. PolyIC (a TLR3 agonist) was less effective than CpG in our study. That result was less useful than we’d like, however, because the common form of polyIC used in human clinical trials for peptide-based cancer vaccines is augmented by the addition of poly-lysine (polyIC-LC). Some trials have also used polyIC-LC plus montanide ISA 51 (a water-in-oil emulsion adjuvant comprising mineral oil and a mannide monooleate surfactant mixed with the antigen peptide).
We are interested in the performance of vaccine formulations as therapeutic tumor antigen vaccines. To this end, we repeated a subset of our prior experiment adding in peptide + polyIC-LC +/- montanide ISA 51 vaccination as treatment groups (Figure 1).
As in our prior experiment, we gave a priming vaccine dose on day 0, a single boost dose on day 14, and then analyzed splenocytes on day 21 for antigen-specific reactivity by interferon gamma ELISPOT. We chose the polyIC-LC dose based on published work, where 50 µg has been routinely administered intramuscularly (Belnoue et al., 2016; Derouazi et al., 2015; Sultan et al., 2020; Tran et al., 2020; Weng et al., 2023; Zhu et al., 2007). We administered polyIC through two injections per time point in the current experiment (as opposed to a single injection per time point in previous experiments) to enable a direct comparison with polyIC-LC, which must be administered through two injections per time point due to the concentration at which this adjuvant is provided and limits on volumes administered intramuscularly in mice.
We also determined the montanide (ISA 51) dose based on published work. Although identification of actual ISA 51 doses used in prior peptide vaccination studies is challenging, a dose of 25-100 µL of ISA 51 mixed 1:1 (v/v) with some other diluent (PBS, peptide suspension, or other adjuvant solution) seems to be common in mice, with 50 µL ISA 51 (prior to 1:1 mixing) being the most frequently used dose (Beebe et al., 2008; Chawla et al., 2019; Chu et al., 2022; Gabri et al., 2006; Ghorbani et al., 2005; Rosenthal et al., 2017; Varypataki et al., 2016). In this experiment, we delivered a total of 50 µL ISA 51 (prior to diluting 1:1) at each vaccine time point. In both humans and mice, this adjuvant is commonly administered subcutaneously or intramuscularly (van Doorn et al., 2015). To be consistent with recent clinical trials (Slingluff et al., 2021), we administered ISA 151 subcutaneously after mixing it 1:1 with peptide suspended in poly-ICLC as per manufacturer recommendations. The preparation of this adjuvant is critical to its success, with the syringe-extrusion/ two-syringe technique recommended over vortexing to mix (Koh et al., 2006; van Doorn et al., 2015). In the current study, we used the syringe-extrusion/two-syringe mixing protocol that was obtained from Seppic, the manufacturer.
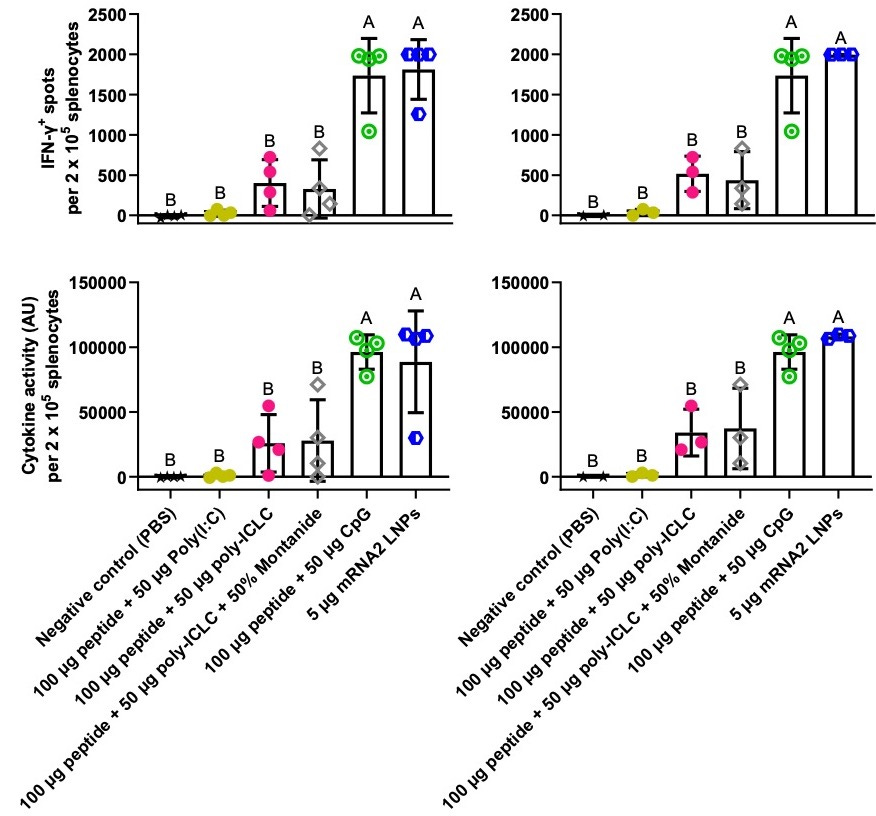
In this experiment, peptide + CpG and mRNA vaccination gave similar results (Figure 2). Although polyIC-LC may have boosted responses relative to polyIC, there was high variance in the polyIC-LC group, and the difference between polyIC and polyIC-LC was not statistically significant in this small study.
Going forward, we will test more vaccine formulations (more adjuvants and DC vaccines next, hopefully DNA vaccines after that), dose titrations of key formulations, different vaccine schedules (varying prime and boost timing and number of injections), and importantly, we will evaluate vaccination against a set of validated neoantigens (rather than the M57 antigen alone) in tumor-bearing mice. Should these results bear out in future studies, we will pursue peptide + CpG and/or mRNA/LNP vaccination for clinical translation.
Many thanks to the Jamie Leandro Foundation (a non-profit foundation dedicated to making therapeutic cancer vaccines available to patients) as well as Natera for funding this work and supporting making the results available on this platform.
References:
Arribillaga, L., Echeverria, I., Belsue, V., Gomez, T., Lozano, T., Casares, N., Villanueva, L., Domingos-Pereira, S., Romero, P. J., Nardelli-Haefliger, D., Hervás-Stubbs, S., Sarobe, P., Rodriguez, M. J., Carrascosa, J. L., Zürcher, T., & Lasarte, J. J. (2020). Bivalent therapeutic vaccine against HPV16/18 genotypes consisting of a fusion protein between the extra domain A from human fibronectin and HPV16/18 E7 viral antigens. Journal for ImmunoTherapy of Cancer, 8(1), e000704. https://doi.org/10.1136/jitc-2020-000704
Barrios, K., & Celis, E. (2012). TriVax-HPV: An improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunology, Immunotherapy: CII, 61(8), 1307–1317. https://doi.org/10.1007/s00262-012-1259-8
Beebe, M., Qin, M., Moi, M., Wu, S., Heiati, H., Walker, L., Newman, M., Fikes, J., & Ishioka, G. Y. (2008). Formulation and characterization of a ten-peptide single-vial vaccine EP-2101, designed to induce cytotoxic T-lymphocyte responses for cancer immunotherapy. Human Vaccines, 4(3), 210–218. https://doi.org/10.4161/hv.4.3.5291
Belnoue, E., Di Berardino-Besson, W., Gaertner, H., Carboni, S., Dunand-Sauthier, I., Cerini, F., Suso-Inderberg, E.-M., Wälchli, S., König, S., Salazar, A. M., Hartley, O., Dietrich, P.-Y., Walker, P. R., & Derouazi, M. (2016). Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-penetrating Peptide-based Vaccines and Adjuvants. Molecular Therapy, 24(9), 1675–1685. https://doi.org/10.1038/mt.2016.134
Cafri, G., Gartner, J. J., Zaks, T., Hopson, K., Levin, N., Paria, B. C., Parkhurst, M. R., Yossef, R., Lowery, F. J., Jafferji, M. S., Prickett, T. D., Goff, S. L., McGowan, C. T., Seitter, S., Shindorf, M. L., Parikh, A., Chatani, P. D., Robbins, P. F., & Rosenberg, S. A. (2020). mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. The Journal of Clinical Investigation, 130(11), 5976–5988. https://doi.org/10.1172/JCI134915
Castro Eiro, M. D., Hioki, K., Li, L., Wilmsen, M. E. P., Kiernan, C. H., Brouwers-Haspels, I., van Meurs, M., Zhao, M., de Wit, H., Grashof, D. G. B., van de Werken, H. J. G., Mueller, Y. M., Schliehe, C., Temizoz, B., Kobiyama, K., Ishii, K. J., & Katsikis, P. D. (2023). TLR9 plus STING Agonist Adjuvant Combination Induces Potent Neopeptide T Cell Immunity and Improves Immune Checkpoint Blockade Efficacy in a Tumor Model. The Journal of Immunology, 212(3), 455–465. https://doi.org/10.4049/jimmunol.2300038
Chawla, B., Mahajan, B., Oakley, M., Majam, V. F., Belmonte, A., Sedegah, M., Shimp, R. L., Kaslow, D. C., & Kumar, S. (2019). Antibody-Dependent, Gamma Interferon-Independent Sterilizing Immunity Induced by a Subunit Malaria Vaccine. Infection and Immunity, 87(10), e00236-19. https://doi.org/10.1128/IAI.00236-19
Chen, J., Ye, Z., Huang, C., Qiu, M., Song, D., Li, Y., & Xu, Q. (2022). Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proceedings of the National Academy of Sciences of the United States of America, 119(34), e2207841119. https://doi.org/10.1073/pnas.2207841119
Cho, H.-I., Barrios, K., Lee, Y.-R., Linowski, A. K., & Celis, E. (2013). BiVax: A peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunology, Immunotherapy: CII, 62(4), 787–799. https://doi.org/10.1007/s00262-012-1382-6
Chu, Y., Qian, L., Ke, Y., Feng, X., Chen, X., Liu, F., Yu, L., Zhang, L., Tao, Y., Xu, R., Wei, J., Liu, B., & Liu, Q. (2022). Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. Journal of Nanobiotechnology, 20(1), 190. https://doi.org/10.1186/s12951-022-01397-7
Derouazi, M., Di Berardino-Besson, W., Belnoue, E., Hoepner, S., Walther, R., Benkhoucha, M., Teta, P., Dufour, Y., Yacoub Maroun, C., Salazar, A. M., Martinvalet, D., Dietrich, P.-Y., & Walker, P. R. (2015). Novel Cell-Penetrating Peptide-Based Vaccine Induces Robust CD4+ and CD8+ T Cell–Mediated Antitumor Immunity. Cancer Research, 75(15), 3020–3031. https://doi.org/10.1158/0008-5472.CAN-14-3017
Gabri, M. R., Mazorra, Z., Ripoll, G. V., Mesa, C., Fernandez, L. E., Gomez, D. E., & Alonso, D. F. (2006). Complete Antitumor Protection by Perioperative Immunization with GM3/VSSP Vaccine in a Preclinical Mouse Melanoma Model. Clinical Cancer Research, 12(23), 7092–7098. https://doi.org/10.1158/1078-0432.CCR-06-1075
Ghorbani, M., Nass, T., Azizi, A., Soare, C., Aucoin, S., Giulivi, A., Anderson, D. E., & Diaz-Mitoma, F. (2005). Comparison of Antibody- and Cell-Mediated Immune Responses After Intramuscular Hepatitis C Immunizations of BALB/c Mice. Viral Immunology, 18(4), 637–648. https://doi.org/10.1089/vim.2005.18.637
Kim, K. Q., Burgute, B. D., Tzeng, S.-C., Jing, C., Jungers, C., Zhang, J., Yan, L. L., Vierstra, R. D., Djuranovic, S., Evans, B. S., & Zaher, H. S. (2022). N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Reports, 40(9), 111300. https://doi.org/10.1016/j.celrep.2022.111300
Koh, Y. T., Higgins, S. A., Weber, J. S., & Kast, W. M. (2006). Immunological consequences of using three different clinical/laboratory techniques of emulsifying peptide-based vaccines in incomplete Freund’s adjuvant. Journal of Translational Medicine, 4, 42. https://doi.org/10.1186/1479-5876-4-42
Liu, J., Lu, X., Li, X., Huang, W., Fang, E., Li, W., Liu, X., Liu, M., Li, J., Li, M., Zhang, Z., Song, H., Ying, B., & Li, Y. (2023). Construction and immunogenicity of an mRNA vaccine against chikungunya virus. Frontiers in Immunology, 14, 1129118. https://doi.org/10.3389/fimmu.2023.1129118
Melssen, M. M., Petroni, G. R., Chianese-Bullock, K. A., Wages, N. A., Grosh, W. W., Varhegyi, N., Smolkin, M. E., Smith, K. T., Galeassi, N. V., Deacon, D. H., Gaughan, E. M., & Slingluff, C. L. (2019). A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. Journal for Immunotherapy of Cancer, 7, 163. https://doi.org/10.1186/s40425-019-0625-x
Panagioti, E., Redeker, A., van Duikeren, S., Franken, K. L., Drijfhout, J. W., van der Burg, S. H., & Arens, R. (2016). The Breadth of Synthetic Long Peptide Vaccine-Induced CD8+ T Cell Responses Determines the Efficacy against Mouse Cytomegalovirus Infection. PLoS Pathogens, 12(9), e1005895. https://doi.org/10.1371/journal.ppat.1005895
Pavlick, A., Blazquez, A. B., Meseck, M., Lattanzi, M., Ott, P. A., Marron, T. U., Holman, R. M., Mandeli, J., Salazar, A. M., McClain, C. B., Gimenez, G., Balan, S., Gnjatic, S., Sabado, R. L., & Bhardwaj, N. (2020). Combined Vaccination with NY-ESO-1 Protein, Poly-ICLC, and Montanide Improves Humoral and Cellular Immune Responses in Patients with High-Risk Melanoma. Cancer Immunology Research, 8(1), 70–80. https://doi.org/10.1158/2326-6066.CIR-19-0545
Ramos da Silva, J., Bitencourt Rodrigues, K., Formoso Pelegrin, G., Silva Sales, N., Muramatsu, H., de Oliveira Silva, M., Porchia, B. F. M. M., Moreno, A. C. R., Aps, L. R. M. M., Venceslau-Carvalho, A. A., Tombácz, I., Fotoran, W. L., Karikó, K., Lin, P. J. C., Tam, Y. K., de Oliveira Diniz, M., Pardi, N., & de Souza Ferreira, L. C. (2023). Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Science Translational Medicine, 15(686), eabn3464. https://doi.org/10.1126/scitranslmed.abn3464
Rosenthal, K. S., Stone, S., Koski, G., & Zimmerman, D. H. (2017). LEAPS Vaccine Incorporating HER-2/neu Epitope Elicits Protection That Prevents and Limits Tumor Growth and Spread of Breast Cancer in a Mouse Model. Journal of Immunology Research, 2017, 3613505. https://doi.org/10.1155/2017/3613505
Sabbatini, P., Tsuji, T., Ferran, L., Ritter, E., Sedrak, C., Tuballes, K., Jungbluth, A. A., Ritter, G., Aghajanian, C., Bell-McGuinn, K., Hensley, M. L., Konner, J., Tew, W., Spriggs, D. R., Hoffman, E. W., Venhaus, R., Pan, L., Salazar, A. M., Diefenbach, C. M., … Gnjatic, S. (2012). Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 18(23), 6497–6508. https://doi.org/10.1158/1078-0432.CCR-12-2189
Schoenmaker, L., Witzigmann, D., Kulkarni, J. A., Verbeke, R., Kersten, G., Jiskoot, W., & Crommelin, D. J. A. (2021). mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. International Journal of Pharmaceutics, 601, 120586. https://doi.org/10.1016/j.ijpharm.2021.120586
Slingluff, C. L., Zarour ,Hassane M., Tawbi ,Hussein Abdul-Hassan, Kirkwood ,John M., Postow ,Michael A., Friedlander ,Philip, Devoe ,Craig E., Gaughan ,Elizabeth M., Mauldin ,Ileana S., Olson ,Walter C., Smith ,Kelly T., Macri ,Mary J., Ricciardi ,Toni, Ryan ,Aileen, Venhaus ,Ralph, & and Wolchok, J. D. (2021). A phase 1 study of NY-ESO-1 vaccine + anti-CTLA4 antibody Ipilimumab (IPI) in patients with unresectable or metastatic melanoma. OncoImmunology, 10(1), 1898105. https://doi.org/10.1080/2162402X.2021.1898105
Sultan, H., Kumai, T., Fesenkova, V. I., Fan, A. E., Wu, J., Cho, H.-I., Kobayashi, H., Harabuchi, Y., & Celis, E. (2018). Sustained Persistence of IL2 Signaling Enhances the Antitumor Effect of Peptide Vaccines through T-cell Expansion and Preventing PD-1 Inhibition. Cancer Immunology Research, 6(5), 617–627. https://doi.org/10.1158/2326-6066.CIR-17-0549
Sultan, H., Wu, J., Fesenkova, V. I., Fan, A. E., Addis, D., Salazar, A. M., & Celis, E. (2020). Poly-IC enhances the effectiveness of cancer immunotherapy by promoting T cell tumor infiltration. Journal for ImmunoTherapy of Cancer, 8(2), e001224. https://doi.org/10.1136/jitc-2020-001224
Tahtinen, S., Tong, A.-J., Himmels, P., Oh, J., Paler-Martinez, A., Kim, L., Wichner, S., Oei, Y., McCarron, M. J., Freund, E. C., Amir, Z. A., de la Cruz, C. C., Haley, B., Blanchette, C., Schartner, J. M., Ye, W., Yadav, M., Sahin, U., Delamarre, L., & Mellman, I. (2022). IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nature Immunology, 23(4), Article 4. https://doi.org/10.1038/s41590-022-01160-y
Temizoz, B., Kuroda, E., Ohata, K., Jounai, N., Ozasa, K., Kobiyama, K., Aoshi, T., & Ishii, K. J. (2015). TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. European Journal of Immunology, 45(4), 1159–1169. https://doi.org/10.1002/eji.201445132
Tran, T.-A.-T., Kim, Y.-H., Duong, T.-H.-O., Jung, S., Kim, I.-Y., Moon, K.-S., Jang, W.-Y., Lee, H.-J., Lee, J.-J., & Jung, T.-Y. (2020). Peptide Vaccine Combined Adjuvants Modulate Anti-tumor Effects of Radiation in Glioblastoma Mouse Model. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.01165
van Doorn, E., Liu, H., Huckriede, A., & Hak, E. (2015). Safety and tolerability evaluation of the use of Montanide ISATM51 as vaccine adjuvant: A systematic review. Human Vaccines & Immunotherapeutics, 12(1), 159–169. https://doi.org/10.1080/21645515.2015.1071455
Varypataki, E. M., Silva, A. L., Barnier-Quer, C., Collin, N., Ossendorp, F., & Jiskoot, W. (2016). Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: A comparative study of cationic liposomes and PLGA nanoparticles. Journal of Controlled Release, 226, 98–106. https://doi.org/10.1016/j.jconrel.2016.02.018
Weng, M.-T., Yang, S.-F., Liu, S.-Y., Hsu, Y.-C., Wu, M.-C., Chou, H.-C., Chiou, L.-L., Liang, J.-D., Wang, L.-F., Lee, H.-S., & Sheu, J.-C. (2023). In situ vaccination followed by intramuscular poly-ICLC injections for the treatment of hepatocellular carcinoma in mouse models. Pharmacological Research, 188, 106646. https://doi.org/10.1016/j.phrs.2023.106646
Xu, K., Lei, W., Kang, B., Yang, H., Wang, Y., Lu, Y., Lv, L., Sun, Y., Zhang, J., Wang, X., Yang, M., Dan, M., & Wu, G. (2023). A novel mRNA vaccine, SYS6006, against SARS-CoV-2. Frontiers in Immunology, 13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.1051576
Yang, J.-X., Tseng, J.-C., Tien, C.-F., Lee, C.-Y., Liu, Y.-L., Lin, J.-J., Tsai, P.-J., Liao, H.-C., Liu, S.-J., Su, Y.-W., Hsu, L.-C., Chen, J.-K., Huang, M.-H., Yu, G.-Y., & Chuang, T.-H. (2023). TLR9 and STING agonists cooperatively boost the immune response to SARS-CoV-2 RBD vaccine through an increased germinal center B cell response and reshaped T helper responses. International Journal of Biological Sciences, 19(9), 2897–2913. https://doi.org/10.7150/ijbs.81210
Zhu, X., Nishimura, F., Sasaki, K., Fujita, M., Dusak, J. E., Eguchi, J., Fellows-Mayle, W., Storkus, W. J., Walker, P. R., Salazar, A. M., & Okada, H. (2007). Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. Journal of Translational Medicine, 5(1), 10. https://doi.org/10.1186/1479-5876-5-10